Category and Product-level Procurement and Delivery Planning Guide
This document lays out indicative lead-times for planning purposes for TB products procured via the Stop TB Partnership’s Global Drug Facility (STBP/GDF).
Procurement and delivery lead-time information is the time from order confirmation and receipt of payment by STBP/GDF to delivery to country. This information is provided at the category and detailed product level and is indicative based on current market knowledge to ensure on-time delivery and best value procurement.
Download the Category and Product-level Procurement and Delivery Planning Guide
STBP/GDF Budgeting Prices for TB Medicines
The Stop TB Partnership's Global Drug Facility (STBP/GDF) has established Long Term Agreements with manufacturers of TB medicines with the aim to achieve lower prices and also ensure the sustainable and reliable on-time supply of the full range of the needed TB medicines.
The prices are indicative prices for budgeting purposes. STBP/GDF aims to deliver the TB medicines at these prices or below. The prices are valid through December 2024.
For more information on the TB medicines please refer to the StopTB/GDF catalog.
Download the STBP/GDF Budgeting Prices for TB Medicines
STBP/GDF Budgeting Prices for TB Diagnostics
The Stop TB Partnership's Global Drug Facility (STBP/GDF) has established Long Term Agreements with manufacturers of TB diagnostics and laboratory supplies with the aim to achieve lower prices and also ensure the sustainable and reliable on-time supply of the full range of needed products.
Pricing as well as information on product specifications, packaging and shipping information can be found in the GDF TB Diagnostics Ordering List. The List also facilitates order planning by automatically calculating order costs and lead times.
For several new TB diagnostic products, guidance on order planning is available in the series of GDF Technical Information Notes.
For an overview of the full range of TB diagnostics and laboratory supplies available through GDF, please refer to the StopTB/GDF catalog.
Indicative Reference Costs for Budgeting Purposes: Freight, Insurance and Quality Assurance
This document lays out the indicative costs for freight, insurance, and quality assurance (QA) for STBP/GDF’s key product categories and is intended for budgeting purposes. The costs are presented as a percentage of product value.
The indicative costs are generalized for all countries and regions. We strongly recommend that you contact your GDF focal point for more tailored guidance on specific countries.
Download the STBP/GDF Indicative Reference Costs for Budgeting Purposes
GDF Estimated Prices for Select Drug-Susceptible TB Treatment Regimens
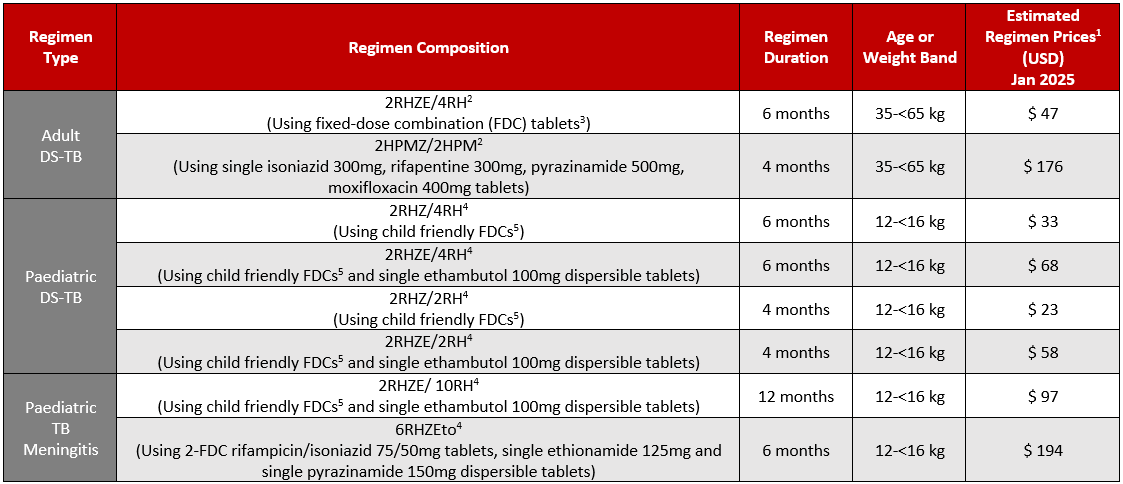
1Estimated regimen prices are calculated using the average weighted price for each medicine (average weighted price accounts for the different prices for each supplier of that medicine weighted by the market share allocation the suppliers received from each GDF tender), the duration indicated (in months) and assuming 30 days of treatment per month. Actual, final prices may differ based on the product(s) delivered.
2Regimen and dosing from WHO operational handbook on tuberculosis Module 4: Treatment – drug-susceptible tuberculosis treatment. Geneva: World Health Organization; 2022. https://www.who.int/publications/i/item/9789240065116
3Using 4-FDC rifampicin/isoniazid /pyrazinamide/ethambutol 150/75/400/275mg and 2-FDC rifampicin/isoniazid 150/75mg tablets)
4Regimen and dosing from WHO operational handbook on tuberculosis. Module 5: management of tuberculosis in children and adolescents. Geneva: World Health Organization; 2022. https://www.who.int/publications/i/item/9789240046832
5Using 3-FDC rifampicin/isoniazid/pyrazinamide 75/50/150mg tablets and 2-FDC rifampicin/isoniazid 75/50mg tablets
Table last updated January 2025
GDF Estimated Prices for Select Drug-Resistant TB Treatment Regimens
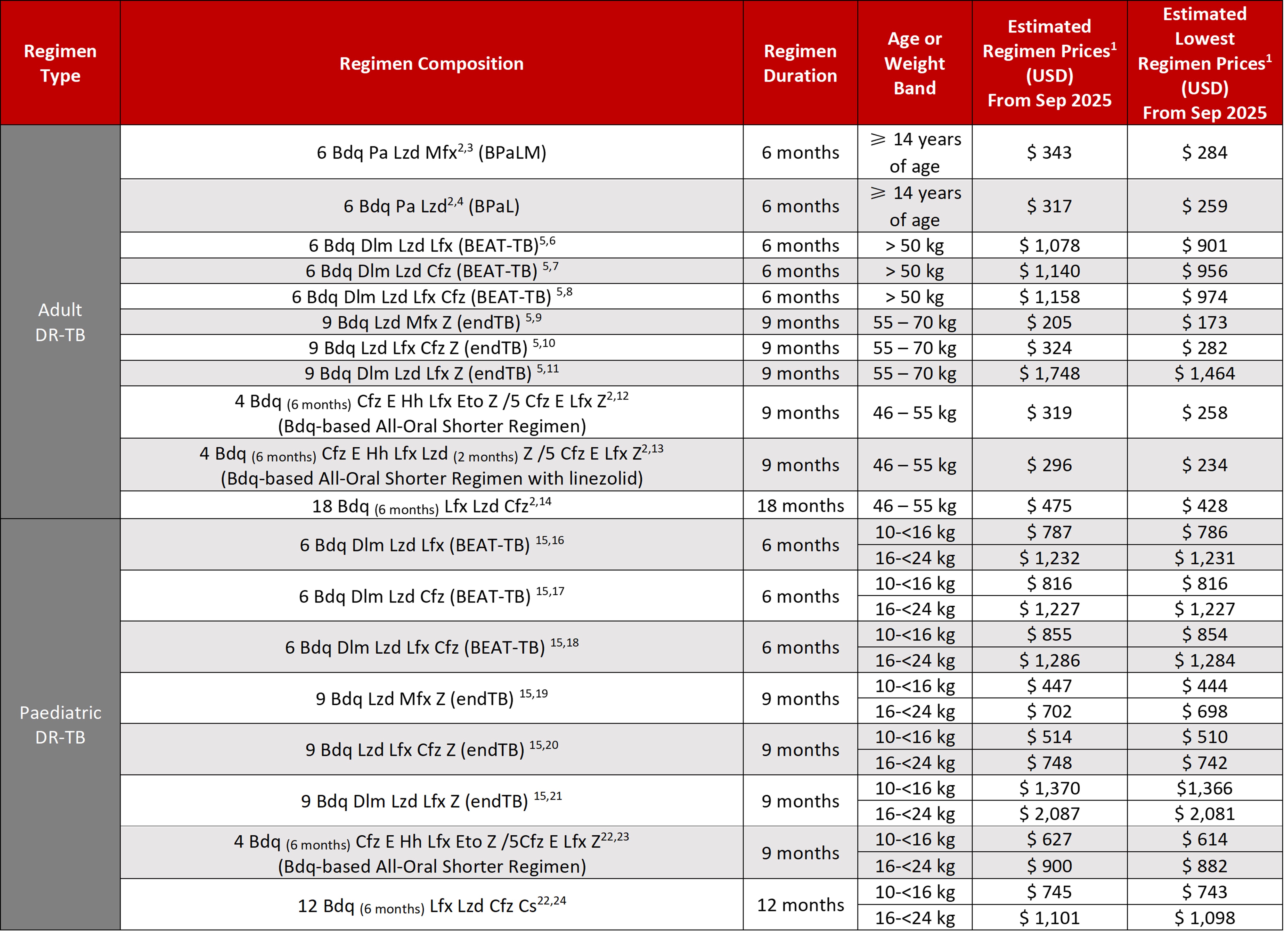
1Estimated regimen prices are calculated using the average weighted price for each medicine (average weighted price accounts for the different prices for each supplier of that medicine weighted by the market share allocation the suppliers received from each GDF tender), the duration indicated (in months) and assuming 30 days of treatment per month. Lowest prices use the lowest price available for that product from all suppliers contracted with GDF, the duration indicated (in months) and assuming 30 days of treatment per month. Actual, final prices may differ based on the product(s) delivered.
2Regimen and dosing from WHO operational handbook on tuberculosis. Module 4: treatment - drug-resistant tuberculosis treatment, 2022 update. Geneva: World Health Organization; 2022 https://www.who.int/publications/i/item/9789240065116
3Using single bedaquiline 100mg, linezolid 600mg, moxifloxacin 400mg and pretomanid 200mg tablets.
4Using single bedaquiline 100mg, linezolid 600mg and pretomanid 200mg tablets.
5Regimen and dosing from Key updates to the treatment of drug-resistant tuberculosis: rapid communication, June 2024. Geneva: World Health Organization; 2024. https://www.who.int/publications/i/item/B09123
6Using single bedaquiline 100mg, delamanid 50g, linezolid 600mg and levofloxacin 250mg tablets.
7Using single bedaquiline 100mg, delamanid 50g, linezolid 600mg and clofazimine 100mg capsules.
8Using single bedaquiline 100mg, delamanid 50g, linezolid 600mg, levofloxacin 250mg tablets and clofazimine 100mg capsules.
9Using single bedaquiline 100mg, linezolid 600mg, moxifloxacin 400mg tablets and pyrazinamide 400mg tablets.
10Using single bedaquiline 100mg, linezolid 600mg, levofloxacin 250mg tablets, clofazimine 100mg capsules and pyrazinamide 400mg tablets.
11 Using single bedaquiline 100mg, delamanid 50g, linezolid 600mg, levofloxacin 250mg tablets and pyrazinamide 400mg tablets.
12 Using single bedaquiline 100mg (for 6 months only), clofazimine 100mg capsules, ethambutol 400mg, isoniazid 300mg, levofloxacin 250mg, ethionamide 250mg, and pyrazinamide 500mg tablets.
13 Using single bedaquiline 100mg (for 6 months only), clofazimine 100mg capsules, ethambutol 400mg, isoniazid 300mg, levofloxacin 250mg, linezolid 600mg (for 2 months only), and pyrazinamide 500mg tablets.
14 Using single bedaquiline 100mg (for 6 months only), levofloxacin 250mg, linezolid 600mg, and clofazimine 100mg capsules.
15 Regimens from WHO Key updates to the treatment of drug-resistant tuberculosis: rapid communication, June 2024. Geneva: World Health Organization; 2024. https://www.who.int/publications/i/item/B09123; dosing from WHO operational handbook on tuberculosis. Module 5: management of tuberculosis in children and adolescents. Geneva: World Health Organization, 2022 https://www.who.int/publications/i/item/9789240046832
16 Using single bedaquiline 20mg dispersible tablet, delamanid 25mg dispersible tablet, linezolid 150mg dispersible tablet, and levofloxacin 250mg dispersible tablet.
17 Using single bedaquiline 20mg dispersible tablet, delamanid 25mg dispersible tablet, linezolid 150mg dispersible tablet, and clofazimine 50mg tablet.
18 Using single bedaquiline 20mg dispersible tablet, delamanid 25mg dispersible tablet, linezolid 150mg dispersible tablet, levofloxacin 250mg dispersible tablet and clofazimine 50mg tablet.
19 Using single bedaquiline 20mg dispersible tablet, linezolid 150mg dispersible tablet, moxifloxacin 100mg dispersible tablet and pyrazinamide 150mg dispersible tablet.
20 Using single bedaquiline 20mg dispersible tablet, linezolid 150mg dispersible tablet, levofloxacin 100mg dispersible tablet, clofazimine 50mg tablet and pyrazinamide 150mg dispersible tablet.
21 Using single bedaquiline 20mg dispersible tablet, delamanid 25mg dispersible tablet, linezolid 150mg dispersible tablet, levofloxacin 250mg dispersible tablet and pyrazinamide 150mg dispersible tablet.
22 Regimen and dosing from WHO operational handbook on tuberculosis. Module 5: management of tuberculosis in children and adolescents. Geneva: World Health Organization, 2022 https://www.who.int/publications/i/item/9789240046832
23 Using single bedaquiline 20mg dispersible tablet (for 6 months only), clofazimine 50mg tablet, ethambutol 100mg dispersible tablet, isoniazid 100mg dispersible tablet, levofloxacin 100mg dispersible tablet, ethionamide 125mg dispersible tablet, and pyrazinamide 150mg dispersible tablet.
24 Using single bedaquiline 20mg dispersible tablet (for 6 months only), levofloxacin 100mg dispersible tablet, linezolid 150mg dispersible tablet, clofazimine 50mg tablet, cycloserine 125mg mini-capsule.
Table last updated September 2025
GDF Estimated Prices for Select TB Preventive Treatment (TPT) Regimen
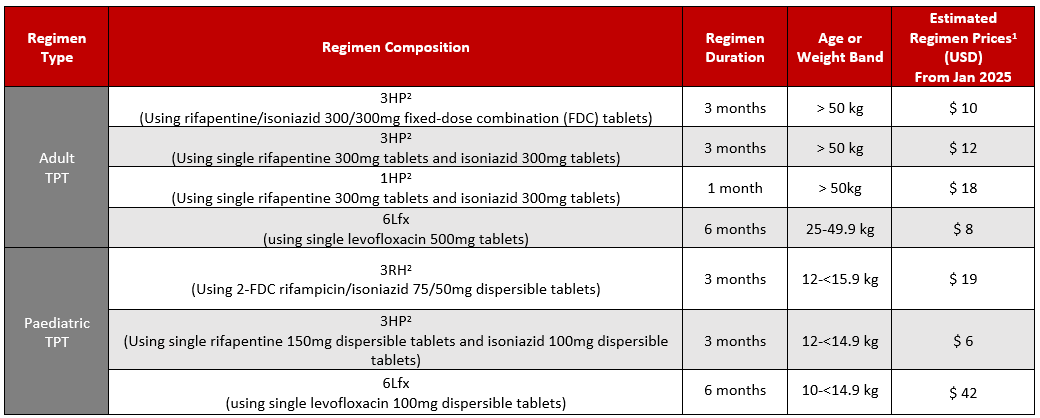
1Estimated regimen prices are calculated using the average weighted price for each medicine (average weighted price accounts for the different prices for each supplier of that medicine weighted by the market share allocation the suppliers received from each GDF tender), the duration indicated (in months) and assuming 30 days of treatment per month. Actual, final prices may differ based on the product(s) delivered.
2Regimen and dosing from WHO operational handbook on tuberculosis. Module 1: prevention - tuberculosis preventive treatment, second edition. Geneva: World Health Organization; 2024. https://www.who.int/publications/i/item/9789240096196
Table last updated January 2025