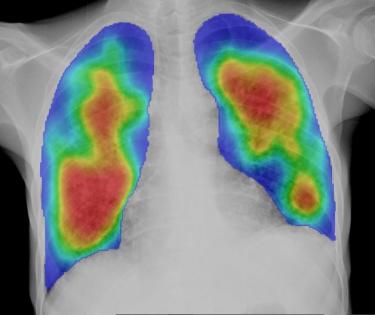
Artificial intelligence (AI) technologies offer unprecedented opportunities within a healthcare context. AI is increasingly being applied in the field of medical imaging for the computer-aided detection (CAD) of diseases, including cancer, COVID-19, and TB.
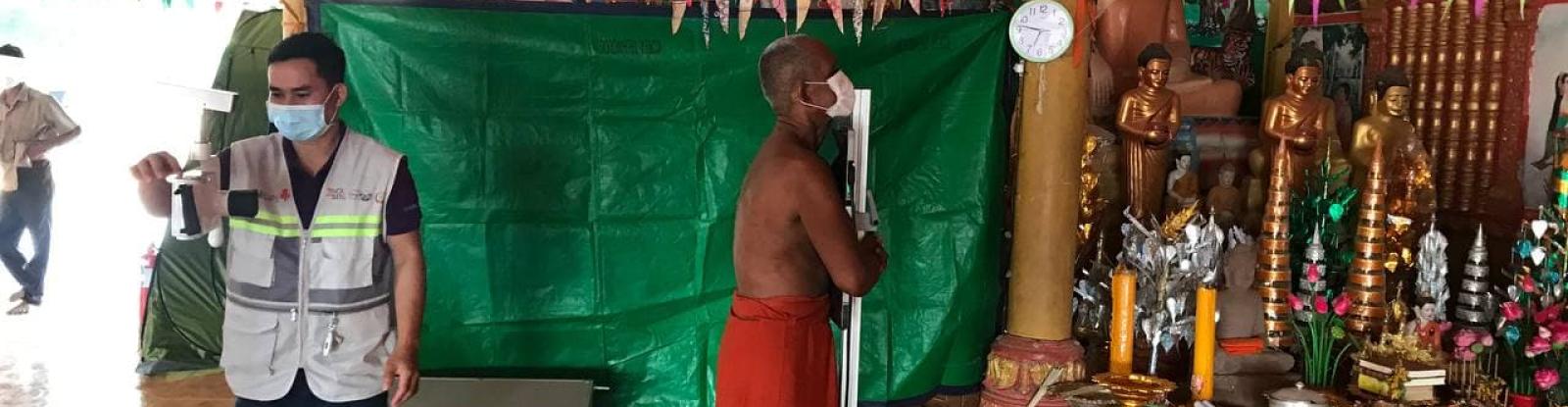
A diverse range of AI products for the recognition of TB-related abnormalities from chest X-rays are now commercially available. Evidence produced by the Stop TB Partnership informed the World Health Organization’s TB screening guideline update in April 2021, when, for the first time, AI was recommended as a triage tool for TB in adults. The potential of AI to accelerate TB detection, particularly in rural and low-resource contexts, is tremendous.
The Stop TB Partnership is working through a vast network of partners to evaluate AI products as an independent body, to effect forward-thinking AI policy and to support implementers to conduct pilot studies and projects. Through the introducing New Tools Project, Stop TB Partnership is leading the largest multi-country implementation of AI and ultra-portable X-ray technology.