Global Health Campus,
Chemin du Pommier 40,
1218 Le Grand-Saconnex, Geneva, Switzerland

Technical publications

PRF diagnostics and MDs (French) - May 2022

Module 5 - Decision maker CAD and X-ray

Time Trend Analysis of Tuberculosis Treatment While Using Digital Adherence Technologies—An Individual Patient Data Meta-Analysis of Eleven Projects across Ten High Tuberculosis-Burden Countries
de Groot LM, Straetemans M, Maraba N, Jennings L, Gler MT, Marcelo D, Mekoro M, Steenkamp P, Gavioli R, Spaulding A, Prophete E, Bury M, Banu S, Sultana S, Onjare B, Efo E, Alacapa J, Levy J, Morales MLL, Katamba A, Bogdanov A, Gamazina K, Kumarkul D, Ekaterina OL, Cattamanchi A, Khan A, Bakker MI.
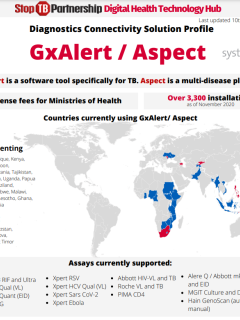
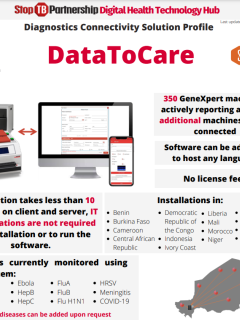

Q&A IDA-GDF-MED-2022-1 - Final

PRF diagnostics and MDs (English) - Apr 2022

Engaging pharmacies in tuberculosis control: operational lessons from 19 case detection interventions in highburden countries
Bigio J, Aquilera Vasquez N, Huria L, Pande T, Creswell J, Ananthakrishnan R, Bimba JS, Cuevas LE, Vo L, Bakker MI, Rahman MT, Pai M.

Wave 10 Additional Resources
Pagination
- Previous page
- Page 25
- Next page