Global Health Campus,
Chemin du Pommier 40,
1218 Le Grand-Saconnex, Geneva, Switzerland
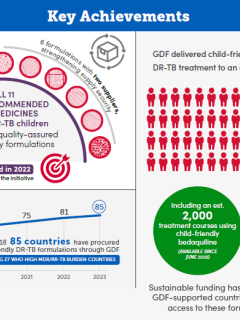
GDF’s Pediatric DR-TB Initiative (EN)

GDF’s Pediatric DR-TB Initiative (EN) - 2

GDF’s Pediatric DR-TB Initiative (JP)

Treatment adherence and clinical outcomes amongst in people with drug-susceptible tuberculosis using medication monitor and differentiated care approach compared with standard of care in South Africa: a cluster randomized trial
Salome Charalambous, Noriah Maraba, Lauren Jennings, Israel Rabothata, Dolphina Cogill, Rachel Mukora, Piotr Hippner, Pren Naidoo, Nokhanyo Xaba, Lihle Mchunu, Kavindhran Velen, Catherine Orrell, Katherine L. Fielding

July 2024 - Urgent Field Safety Notice concerning BD BACTEC™ MGIT™ 960 PZA Kit manufactured by BD

TPMAT Recommendations - Round 29 (EOI Released July 2024)

Annex L. Declaration of supply restrictions to countries DLM ITB-Iplus/GDF-MED/2024/1

Annex K. Form for Requests for Clarifications or Complaints after ITB awarding DLM ITB-Iplus/GDF-MED/2024/1

Annex J. GDF packaging artwork development guidelines DLM ITB-Iplus/GDF-MED/2024/1

Annex I. GMSD address list DLM ITB-Iplus/GDF-MED/2024/1
Pagination
- Previous page
- Page 7
- Next page