29 June 2015 - India, the second highest populous country in the world -- accounts for the highest share of TB incidence cases globally. Among its 1.2 billion population, 2.1 million TB cases are estimated to occur annually. This translates to a quarter of the global TB incidence. In order to effectively address this challenge, the country implemented the Revised National Tuberculosis Control Programme (RNTCP) in 1997 achieving complete national coverage in the year 2006. The RNTCP detects and treats approximately 1.5 million cases annually. TB elimination however is not achievable unless drug-resistant TB - the most critical challenge in recent times -- is not effectively addressed. Diagnosis and the management of drug-resistant TB poses greater challenges due to the complexity of diagnosis, its long duration in treatment and the potentially toxic drugs. Additionally, the high cost in resources, the transformation of clinical management into public health management settings, and the need for specialists’ support for management make it even more challenging. The annual estimated number of multidrug-resistant (MDR-TB) cases among the notified pulmonary TB cases is 61,000 -- which is the highest among all countries.
Programmatic Management of Drug Resistant Tuberculosis (PMDT) services were rolled out in the country in 2007. The targets for PMDT expansion were to establish 43 laboratories for diagnosis and 120 specialized centres for treatment of MDR-TB. Annual enrolment of at least 40,000 MDR-TB patients for treatment by 2017 on full scale up was an ambitious target. The smallest unit in India’s population scales for presumptive and confirmed TB cases is in the millions, and in the thousands for the presumptive and confirmed MDR-TB/XDRT-B cases. Expanding access to the entire population is therefore a herculean task. Geographic, ethnic, socio-cultural, socio-economic, behavioural, and health infrastructure diversities of this united largest democracy were perceived as inherent, but not unbeatable challenges for this expansion.
Expanded PMDT services to achieve nation wide coverage
Sustained politico-administrative commitment, pragmatic scale up plans, need-based and result-oriented deployment of resources and effective monitoring at all levels resulted in the nationwide expansion of PMDT services providing access to the entire population. After a period of slow progress since its roll out in 2007, the PMDT expansion was dramatically accelerated from 2011, to achieve nationwide coverage in 2013. It started with a consultative process in 2010, assessing the resources, setting targets, milestones and priorities for each state. Ambitious domestic funding, with additional support from The Global Fund, WHO, UNITAID and USAID took care of the funding needs and high end technical assistance was provided by WHO, National Institutes and partner agencies. WHO approved rapid molecular diagnostics like Line Probe Assay (LPA), which takes only two days and Cartridge Based Nucleic Acid Amplification Test (CBNAAT) which takes only two hours for the result. These were deployed to address the lab capacity deficit and early and faster diagnosis of drug-resistant TB cases. Four national training centres were set up to address the need for human resource capacity building. Training was imparted to the whole program staff on a cascade model, to ensure capacity for identification of drug-resistant TB patients, as well as the collection of paired sputum samples, packaging and transportation to the designated DST laboratory. Logistics for sample collection and transportation, & recording and reporting were ensured in each district. Districts were appraised to assess the preparedness before a final go-ahead for roll out in a phased manner was given. Although these activities were resource intensive, persistent planning and implementation produced the desired results.
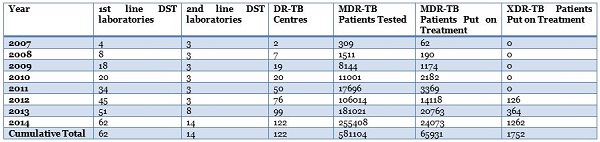
Since then, PMDT has made steady progress. Annual physical achievements are tabulated below.
Expansion of diagnostic drug susceptiability testing (DST) services to patients with less graded risk of drug resistance
During the early implementation years, drug susceptability testing was offered to patients failing a first line treatment regime. Hence patients having drug resistance at the beginning or early in the treatment could not access this service. Along with scale up of laboratory capacity, PMDT has now made diagnostic services available to TB patients at the earliest during the treatment. Additionally, TB patients who are contacts of MDR-TB cases, HIV infected TB patients and treatment experienced TB patients are being offered DST at baseline. It has contributed to early diagnosis, reduction in airborne infection and increased treatment adherence.
Deployment of rapid molecular diagnostic technologies for effective expansion
Initially PMDT deployed diagnostic mechanism based on culture in solid medium followed by DST. This took three to four months to get the DST report after identification of an MDR-TB suspect. As a consequence, a significant proportion of MDR-TB patients died or opted treatment outside the program. WHO-approved rapid molecular diagnostics were deployed by the country during 2011-12. A total of 43 labs with LPA and 80 CBNAAT machines were deployed under PMDT to ensure all districts in the country have access to rapid diagnostics.
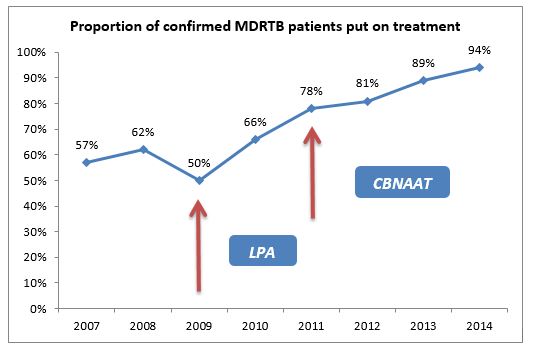
Remarkable improvement in the proportion of diagnosed MDR TB patients who were put on treatment as an outcome of PMDT expansion
A significant proportion of MDR-TB patients were not being put on treatment during the initial implementation years, due to the long processing period for Solid Culture & DST on one hand, and suboptimal management capacity in the field to track the patients and put on treatment. With deployment of rapid molecular diagnostics, and efforts to improve program management capacity in the field, this gap narrowed and most of the MDRTB patients diagnosed were put on treatment in recent years, as depicted in the chart below. Year 2014 reported the highest of 94% among diagnosed MDR-TB cases initiated on second line regimen.
Deployment of Second Line DST for diagnosis and management of Extensively Drug Resistant TB (XDRTB)- next service to expand countrywide
XDR-TB, defined as resistance to Quinolone group and injectable second line drugs, requires more sophisticated diagnostic techniques and more complex treatment. PMDT could develop 11 additional laboratories including three in the non-governmental sector; to have 14 engaged in the programme. 1752 XDR-TB patients have been put on treatment in the past four years. The second-line DST services are being rapidly expanded in India to provide linkage to all districts in order to finally achieve access to second-line DST at baseline.
Having over achieved all planned targets, the programme has since committed to moving towards universal Drug Susceptibility Testing (DST) and DST guided treatment. The programme now has plans to scale up laboratory capacity to 120 for first line drugs, 40 for second line drugs and to have in place a cartridge-based nucleic acid amplification (CBNAAT) machine in each district and medical college by 2017.
Decentralization of treatment services under PMDT
The National TB Programme rolled out treatment services with only two drug-resistant centres in the states of Gujarat and Maharashtra. A drug-resistant centre was established for every 10 million population over the last four years and there is a target of 120 drug-resistant TB centres by 2017. India has achieved this three years ahead of schedule. The country now plans the decentralization of PMDT treatment services up to district level with a drug-resistant centre at every district over next three years.