22 August 2018 | GENEVA - The Stop TB Partnership welcomes the World Health Organization’s (WHO) release of a Rapid Communication: Key changes to the treatment of multidrug- and rifampicin-resistant tuberculosis (MDR/RR-TB). The changes are based on recommendations of the WHO Guideline Development Group which met in July 2018.
The new rapid communication marks the first time in history that WHO recommends injection-free regimens for the treatment of MDR-TB. This new WHO announcement follows shortly after a bold move by the government of South Africa to adopt a nation-wide transition to the injectable-free treatment of DR-TB.
WHO’s rapid communication aims to provide information and advice on immediate steps to be taken to ensure that people with MDR/RR-TB receive treatment in accordance with the latest evidence on the effectiveness and safety of the medications available.
The full guideline update is expected to be released later in 2018.
WHO’s announcement is timely as heads of state, government ministers, parliamentarians, UN leaders, civil society, TB community representatives, the private sector, academics and many others prepare to attend the first ever United Nations High-Level Meeting on TB in September 2018.
The meeting is expected to endorse a Political Declaration that includes ambitious milestones and targets, including expectations to diagnose and treat at least a cumulative number of 1.5 million people with MDR-TB by the end of 2022.
The rapid communication calls for a re-grouping and reprioritization of the medicines used to treat MDR/RR-TB, including:
The WHO changes have huge and significant implications for all people with MDR-TB - a vast majority of which will benefit, for the first time, from an injection-free regimen with fewer side effects.
These changes also have implications for national TB programmes - on planning (several areas but especially in procurement and supply), human resources, financing, implementation and monitoring of the TB response. WHO advises countries to rapidly adjust their national treatment policies, drug procurement plans and monitoring system to quickly switch to the new priority regimens. As it is not possible to effect these changes immediately, national TB programmes should immediately develop transition plans to address these critical issues.
PLEASE NOTE: Lead times for new medicine orders are up to 6 months from the time orders are finalized.
A. Changes in demand for medicines used in new MDR-TB regimens
The rapid communication will dramatically impact the demand for most medicines used to treat MDR-TB as portrayed in Table 1 below.
B. Changes in prices for new MDR-TB regimens
The new, all oral, 20-month MDR-TB regimens range from US $1,600* (using bedaquiline and linezolid for 6 months and levofloxacin as the fluoroquinolone) to US $2,100* (using linezolid for 12 months and moxifloxacin as the fluoroquinolone). See below Table 2 with price estimations for newly recommended MDR-TB regimens, based on Stop TB`s GDF Medicines Catalog and prices. For more details, please contact gdf@stoptb.org.
PLEASE NOTE: These new all-oral regimens are approximately 2- to 3-times more costly than the previously recommended longer regimens for MDR-TB (StopTB GDF Announcement on Price Reductions June 2018).
C. Changes in TB diagnostics
The new WHO rapid communication will require expanded laboratory capacity given the critical role that drug-susceptibility testing (DST) plays in ensuring patients are put on the most effective treatment regimen. Stop TB`s GDF provides a wide range of diagnostic equipment and laboratory supplies in its Diagnostics Catalog. Key diagnostic messages from the new rapid communication include:
Stop TB’s GDF continues to serve as a one-stop shop for TB medicines and diagnostics.
All country programmes are eligible to access quality-assured medicines and diagnostics from GDF.
What we offer:
Table 1. Expected changes in demand for medicines used to treat MDR-TB**
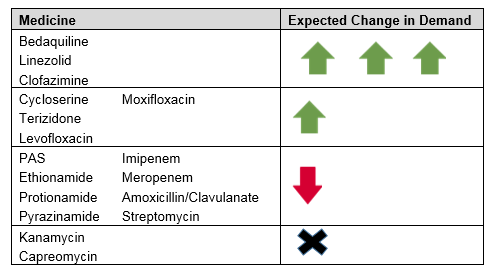
**Expected trends for other medicines (i.e., ethambutol, amikacin, delamanid) are unclear.
Table 2. 2018 GDF estimated prices* for new MDR-TB Regimens
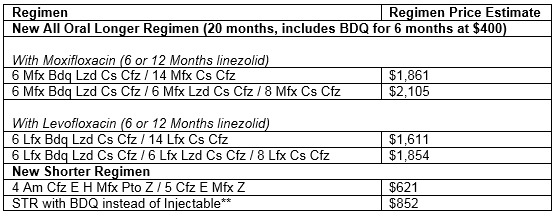
*Weighted average according to volumes allocated to suppliers in 2018 tender
** As per WHO recommendations on operational research
For more information, please contact gdf@stoptb.org.